This is my thought process on how and why I built my hot air injection vapor system.
After reading about "Smokey Yunick's Hot Vapor Fiero" I thought that there must be some truth to utilizing fuel in a vapor form.
Today's DI test engines are now seeing major improvements in a fuel vapor form, and as Mech as mentioned "transonic combustion" has improved even more on this by adding a fuel heater to help with the DI soot problem. So the questions now is how does a back yard mechanic get there.
My pour test in the above pics, showed me a few things. The biggest visual is the distillation curve of today's pump gasoline is way different then yesterdays. The very slow evaporation rate of the pump gasoline stands out big time!!! So I had to do some fact finding on what and why is today's fuel so slow to evaporate.
GASOLINE REQUIREMENTS AND PROPERTIES
In order to maintain top road performance and best fuel economy gasoline must be formulated and blended to provide:
Good mileage under all driving conditions
Fast starts
Rapid warm-up
Smooth performance
Minimum engine maintenance
There are three major factors which govern the performance characteristics of gasolines:
Volatility
Anti-knock quality
Deposit control
Volatility
This is a measure of the tendency of a gasoline to change from a liquid to a vapour. The distillation curve (Diagram A-1) of a gasoline is an indication of its volatility and is obtained by a distillation test (ASTM D-86). Another measure of gasoline volatility is its Reid Vapour Pressure. RVP is a measure of fuel vapour pressure at 38°C under specified conditions (ASTM D-323). It is largely a measure of the butane content of the gasoline.
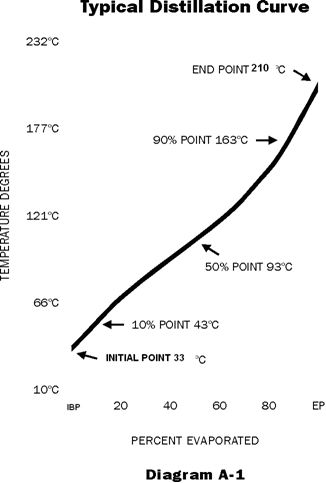
There are a number of performance characteristics affected by volatility:
- Cold starting
- Vapour lock
- Fuel system icing
- Warm up and acceleration
- Economy (especially short trips)
- Combustion chamber deposits
- Crankcase dilution
2. Starting and Vapour Lock Protection
These gasoline properties are directly opposed and it is necessary to strike a compromise in the interest of over-all fuel performance. Starting ability is controlled by the Reid Vapour Pressure and the front end of the distillation curve. (10 - 20 - 30% temperatures) Fortunately, during the winter months, when quick starting is important, it is possible to blend in adequate front end volatility for starting without exceeding the desired vapour lock protection temperatures. (As shown in Diagram A-2)
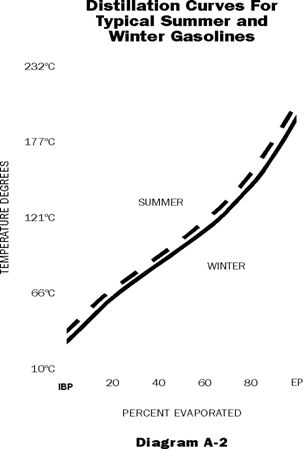
Conversely during the summer months, vapour lock protection becomes important, and the somewhat less volatile gasoline distributed in the warm weather provide this protection without sacrificing starting ability. Spring and fall call for careful adjustment of front end volatility to make the best compromise.
It should be kept in mind that there is an exchange between RVP and front end distillation values and that vapour locking tendency should not be judged by RVP alone.
Gasoline volatility is adjusted seasonally. For optimum performance in all gasoline engines, it is best to use fuel which is blended for the season in which it is used.
3. Engine Warm-Up and Acceleration
The effect of fuel volatility on engine's performance is quite marked and is related to the 50% and 90% evaporated temperatures (or to the % at 100°C and 160°C). In general, the lower these temperatures are, the faster the engine will warm up. This is because once the engine starts, the temperature of its induction system must rise to a point where sufficient gasoline is vaporized to obtain good mixture distribution to all cylinders, and to permit full throttle acceleration without hesitation or "bucking" without choke. This is defined as warm-up time.
The trend has been towards lower 50% and 90% temperatures, since the motorist readily detects good warm-up and acceleration. Another advantage of a low 90% evaporated temperature is the reduced tendency for crankcase dilution.
4. Fuel Economy
The carburetor or fuel injection air-fuel setting has a major effect on gasoline consumption. If it is richer than necessary the consumption will be high. The general relationship between air-fuel ratio, power and fuel economy, is illustrated in Diagram A-3.
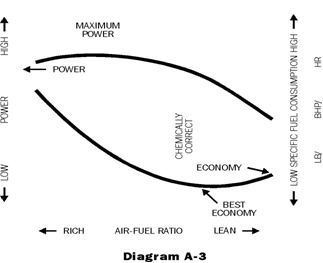
The driver and driving conditions are important factors in fuel economy. Also the useable energy content of the fuel is significant. The higher boiling components have higher energy contents per litre, thus the backend of the distillation range is a measure of the energy content of the fuel. However, the higher boiling components contribute most to crankcase oil dilution and to some degree to combustion chamber deposits. A compromise between economy, oil dilution, and deposits is necessary.
Surveys show that about 2/3 of all driving consists of trips of 10 kilometres or less. For such short trip driving, particularly in winter when engine temperature is lower, the more volatile fuel with a greater mid fill volatility gives greater economy.
5. Anti-Knock Quality (Octane Number)
Increasing the pressure of the fuel mixture in the combustion chamber before ignition helps to increase power of an engine. This is done by compressing the fuel mixture to a smaller volume. Higher compression ratios not only boost power but also make for more efficient power. But as the compression ratio goes up, knocking tendency increases and the anti-knock value of the fuel becomes critical.
The octane number of a gasoline is a measure of its anti-knock quality or ability to resist detonation during combustion. Detonation, sometimes referred to as "spark knock" or "ping" can be defined as an uncontrolled explosion of the last portion of the burning fuel-air mixture due to excessive temperature and pressure conditions in the combustion chamber. Since detonation creates shock pressure waves, hence audible knock, rather than smooth combustion and expansion of the fuel-air mixture, it results in loss of power, excessive localized temperatures, and engine damage if sufficiently severe.
There are two commonly used methods of determining the octane number of gasoline, the Motor Method and the Research Method. Both use the same type of laboratory single cylinder engine, which is equipped with a variable head and a knock-meter to indicate knock intensity. Using the test sample as fuel, the engine compression ratio and the air/fuel are adjusted to develop a specified knock intensity. Two primary standard reference fuels, normal heptane and iso-octane, arbitrarily assigned 0 and 100 octane numbers respectively, are then blended to produce the same knock intensity as the test sample. The percentage of iso-octane in the blend is considered the octane number of the test sample. Thus, if the matching reference blend is made up of 15% n-heptane and 85% iso-octane, the test sample is rated 85 Motor or Research octane number, according to the test method used.
The test conditions for the Research Method are less severe than for the Motor Method. Accordingly commercial gasolines have a higher octane number by the Research Method than by the Motor Method.
Both ratings are required to predict the performance of the gasoline in actual road usage, but the relative importance depends on the type of engine and operating conditions. In truck and farm equipment operation where the gasoline must perform at high temperatures and under maximum throttle, the Motor Octane Number (MON) is more significant than the Research Octane Number (RON). Similarly in passenger cars with automatic transmissions under full and part throttle the Motor Octane Number (MON) is more significant, particularly in engines of recent manufacture. In passenger cars with manual transmissions at full throttle where the engine can be loaded at low engine speed the Research Octane Number (RON) is more significant than the Motor Octane Number (MON), while at part throttle the reverse is true.
Road Octane Number is the rating of the gasoline in terms of reference fuels, by a test car under full throttle acceleration from 10 to 50 mph. However, Road Octane tests are too expensive for routine testing and are reserved mainly for special octane requirement surveys. An approximation may be made by calculation of the average of Research and Motor Octane Number:
(RON) + (MON)
2
This calculation is known as the Anti-Knock Index and correlates with a gasoline expected "Road" Octane Number requirements.
For our purposes in keeping our members better informed, the Anti-Knock Index is perhaps the most important reference to Octane. The Anti-Knock Index is commonly referred to in vehicle operator's manuals and is on gasoline pumps in the U.S., by law.
There are a number of factors that affect octane requirement in addition to compression ratio (as shown in Diagram A-4):
Another important fact to know in regards to anti-knock quality is that nothing is gained or lost by using gasoline of higher octane quality than required by the engine in the whole range of its operating conditions. The full value of higher octane gasoline is only obtained when an engine is designed and adjusted to take advantage of it. Most engines are satisfied with the "regular" grade gasoline, but those that are not satisfied can use a "mid" or "premium grade".
GASOLINE ADDITIVES
The following is a list of gasoline additives showing properties and uses:
1. Oxidation Inhibitors
Gasoline stability is important since gums formed during storage by the reaction of some gasoline components with each other and with oxygen may result in harmful deposits in the engine. Oxidation inhibitors are added to help in controlling gum and deposit formation.
2. Metal Deactivators
Reactions between the fuel and the metals in the fuel system may form abrasive and filter plugging substances. Metal deactivator additives are used to inhibit such reactions.
3. Detergents
Detergent additives are used to keep carburetor and fuel injection system parts clean and allow for the designed functioning of these engine components.
4. Rust Inhibitors
Fuel system parts are protected by the addition of rust inhibitors.
5. Octane Enhancers
Since the ban of lead in auto gasoline as of December 1, 1990, refiners are required to use alternate compounds to improve the anti-knock quality of the gasoline. MMT (methylcyclopentadienyl manganese tricarbonyl) is a catalyst compatible octane improver.
UFA GASOLINE ADDITIVES
All blends and grades of gasolines marketed by UFA are fortified with a comprehensive additive system.
These gasoline additives have been specifically designed to precisely alleviate the type of fuel related problems associated with fuel injectors and carburetors. By removing and preventing deposits, it restores and maintains peak engine performance.
Many benefits can be achieved through regular uninterrupted use of UFA gasoline, which is fortified with a unique additive system.
- Smoother acceleration
- Improved warm-up and idling performance
- Protection against rust
- Elimination of emulsion problems
- Improved fuel economy
- Increased engine reliability
As shown in Diagram A-5 fuel injectors must be deposit free in order to provide an even fuel flow pattern. UFA gasoline can eliminate deposits which lead to fouled fuel injectors.
All UFA gasoline contains sufficient deposit control additive to meet ASTM D5500, which requires an intake valve deposit of less than 100 mg average deposit mass per valve after a 16,093 kilometre (10,000 mile) driving cycle, or less than 25 mg average deposit mass per valve after a 8,046 kilometre (5,000 mile) driving cycle.
Source:
Gas fuel characteristics and resources