Quote:
Originally Posted by IamIan

Are you claiming that temperature alone is the only information needed to determine what the expansion ratio will be for saturated steam? ... that no other factors of the context will have any influence at all???
|
That's right.
Like I claimed earlier in this tediously long thread, I said that water is a strange critter.
Quote:
Originally Posted by IamIan

II am also getting the impression you seem to hold that the existence of steam tables somehow nullifies or contradicts the relationships shown in the Ideal Gas Law ... I disagree with this ... No gas is actually ideal ... steam tables are just the measured results of carrying out experiments under the listed conditions ... it in no way changes or nullifies the relationships shown in the Ideal Gas Law ... the most it might do is tweak the resolution of some of the results... but one must keep in mind the context of those tables, to keep their shown values properly applied.
|
1. You're quibbling about the ideal gas law. While it is true that no gas perfectly behaves as an ideal gas, the ideal gas law is sufficient for most gases. Not saturated steam, and not for steam in various regions of temperature and pressure.
2. You're flat-out wrong about using "since no gas exactly follows the ideal gas law, we can still treat steam as an ideal gas."
Here:
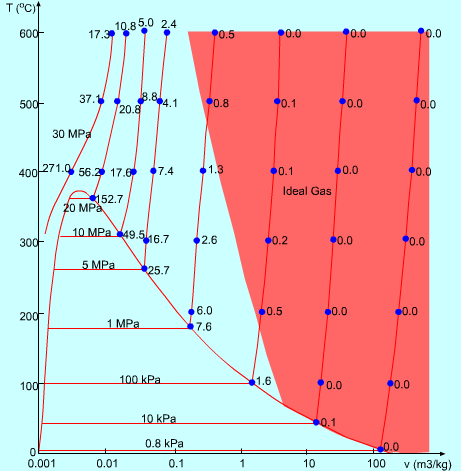
The red area shows where you can treat steam as an ideal gas. Note that for most of the saturation line between steam and liquid, there is no red.