 04-24-2012, 07:45 PM
04-24-2012, 07:45 PM
|
#131 (permalink)
|
|
MPGuino Supporter
Join Date: Oct 2010
Location: Hungary
Posts: 1,808
iNXS - '10 Opel Zafira 111 Anniversary Suzi - '02 Suzuki Swift GL
Thanks: 831
Thanked 710 Times in 458 Posts
|
Quote:
Originally Posted by IamIan

Are you claiming that temperature alone is the only information needed to determine what the expansion ratio will be for saturated steam? ... that no other factors of the context will have any influence at all???
|
That's right.
Like I claimed earlier in this tediously long thread, I said that water is a strange critter.
Quote:
Originally Posted by IamIan

II am also getting the impression you seem to hold that the existence of steam tables somehow nullifies or contradicts the relationships shown in the Ideal Gas Law ... I disagree with this ... No gas is actually ideal ... steam tables are just the measured results of carrying out experiments under the listed conditions ... it in no way changes or nullifies the relationships shown in the Ideal Gas Law ... the most it might do is tweak the resolution of some of the results... but one must keep in mind the context of those tables, to keep their shown values properly applied.
|
1. You're quibbling about the ideal gas law. While it is true that no gas perfectly behaves as an ideal gas, the ideal gas law is sufficient for most gases. Not saturated steam, and not for steam in various regions of temperature and pressure.
2. You're flat-out wrong about using "since no gas exactly follows the ideal gas law, we can still treat steam as an ideal gas."
Here:
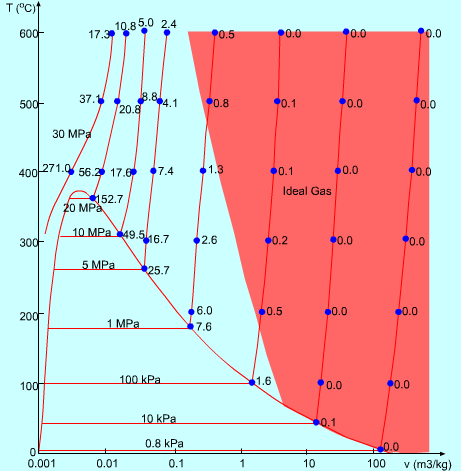
The red area shows where you can treat steam as an ideal gas. Note that for most of the saturation line between steam and liquid, there is no red.
|
|
|

|
 Today Today
|
|
|
|
 Other popular topics in this forum...
Other popular topics in this forum...
|
|
|
|
 04-24-2012, 08:26 PM
04-24-2012, 08:26 PM
|
#132 (permalink)
|
|
Corporate imperialist
Join Date: Jul 2011
Location: NewMexico (USA)
Posts: 11,312
Thanks: 273
Thanked 3,584 Times in 2,845 Posts
|
You never ever, ever use ideal gas law for steam.
Use steam tables, they have been established for over 150 years and have been revisited, occasionally revised over the years and expanded.
Unless you are working out side established tables then there is not much choice.
__________________
1984 chevy suburban, custom made 6.5L diesel turbocharged with a Garrett T76 and Holset HE351VE, 22:1 compression 13psi of intercooled boost.
1989 firebird mostly stock. Aside from the 6-speed manual trans, corvette gen 5 front brakes, 1LE drive shaft, 4th Gen disc brake fbody rear end.
2011 leaf SL, white, portable 240v CHAdeMO, trailer hitch, new batt as of 2014.
|
|
|

|
 04-24-2012, 08:46 PM
04-24-2012, 08:46 PM
|
#133 (permalink)
|
|
Banned
Join Date: Feb 2008
Location: california
Posts: 1,329
Thanks: 24
Thanked 161 Times in 107 Posts
|
|
|
|

|
 04-25-2012, 10:50 AM
04-25-2012, 10:50 AM
|
#134 (permalink)
|
|
Master EcoModder
Join Date: Dec 2011
Location: Boise Idaho
Posts: 842
Thanks: 39
Thanked 89 Times in 69 Posts
|
what is the temperature of combustion?
I'm assuming it is safely north of 800c.
which means we treat it as an ideal gas.
to figure out the temperature of the water, we need the btu's of the system if we want to find equilibrium.
|
|
|

|
 04-25-2012, 01:22 PM
04-25-2012, 01:22 PM
|
#135 (permalink)
|
|
Corporate imperialist
Join Date: Jul 2011
Location: NewMexico (USA)
Posts: 11,312
Thanks: 273
Thanked 3,584 Times in 2,845 Posts
|
Its not above 800C the entire time.
On a diesel, where water injection is proven to work, combustion tempatures can hit 3,000F+ at the peak but exhaust gas temperatures drop to 400-800F during cruise.
Real engineers dont use ideal gas for steam.
After me and my engineer buddies got lost in steam tables, ideal gas law, studying the lubercation properties of steam/water to figure out why water injection boosts fuel economy and power of diesels and gas turbines we setteled on this:
Water injection - EcoModder
Remember this is for a diesel, I am still convinced that for about 95% of the time when you try to run water injection continiously during cruise on a factory production (Original Equipment) gas engine you are going to hurt fuel economy.
Its going to take a lot of work, lots of testing and lots of tuning to produce a positive result on OE gas burners. But you can try.
I bet I can do it, but don't have a way to test it, yet.
__________________
1984 chevy suburban, custom made 6.5L diesel turbocharged with a Garrett T76 and Holset HE351VE, 22:1 compression 13psi of intercooled boost.
1989 firebird mostly stock. Aside from the 6-speed manual trans, corvette gen 5 front brakes, 1LE drive shaft, 4th Gen disc brake fbody rear end.
2011 leaf SL, white, portable 240v CHAdeMO, trailer hitch, new batt as of 2014.
|
|
|

|
 04-25-2012, 02:10 PM
04-25-2012, 02:10 PM
|
#136 (permalink)
|
|
MPGuino Supporter
Join Date: Oct 2010
Location: Hungary
Posts: 1,808
iNXS - '10 Opel Zafira 111 Anniversary Suzi - '02 Suzuki Swift GL
Thanks: 831
Thanked 710 Times in 458 Posts
|
Quote:
Originally Posted by oil pan 4

After me and my engineer buddies got lost in steam tables, ideal gas law, studying the lubercation properties of steam/water to figure out why water injection boosts fuel economy and power of diesels and gas turbines we setteled on this:
Water injection - EcoModder |
That is correct. The theoretical maximum efficiency for a Diesel cycle engine depends both on the compression ratio, and on the inlet and combustion temperatures. Lower the inlet temperature, such as with flash evaporation of a fine water mist, and Diesel efficiency goes up.
The theoretical maximum efficiency for the Otto cycle (gasoline) engine depends solely on its compression ratio. Lower the temperature of the inlet air, and the efficiency is left unchanged.
Quote:
Originally Posted by oil pan 4

Its going to take a lot of work, lots of testing and lots of tuning to produce a positive result on OE gas burners. But you can try.
I bet I can do it, but don't have a way to test it, yet.
|
Aside from the obvious benefit of moving the conditions of the combustion chamber away from detonation and pre-ignition, hot water injection could be a way to permit throttling with a lower intake manifold vacuum. Normally, gasoline engines require the use of a vacuum in order to throttle the air going into them. High vacuums take energy to create and maintain, and do not actually push the vehicle down the road. Higher vacuums require a larger percentage of engine power to maintain, than do lower vacuums. If hot water injection were used in this instance, flash evaporation of the water could contribute a pressure increase inside the intake manifold, while leaving the oxygen content of the intake manifold unchanged. This would allow the engine to use less of its developed power to maintain the intake manifold vacuum, and use more of it to actually propel the vehicle down the road.
Now, inject too much water, and you run into ignition quenching issues. Inject more water than that, and you run the risk of hydrolocking the engine. I'm not so concerned about water vapor in the combustion chamber possibly leaking out and contaminating the oil, since exhaust has plenty of water vapor in it already.
This was what I was hoping to see with tjts1's logs. |
|
|

|
 04-25-2012, 03:34 PM
04-25-2012, 03:34 PM
|
#137 (permalink)
|
|
Corporate imperialist
Join Date: Jul 2011
Location: NewMexico (USA)
Posts: 11,312
Thanks: 273
Thanked 3,584 Times in 2,845 Posts
|
Quote:
Originally Posted by t vago

The theoretical maximum efficiency for the Otto cycle (gasoline) engine depends solely on its compression ratio. Lower the temperature of the inlet air, and the efficiency is left unchanged.
|
Yeah you nailed it.
This is how I would use water alcohol injection.
Build the engine to have a fairly high compression ratio, up to around 12.5:1 with a factory or mild preformance cam. The engine would run like any other engine out there, nothing special. Normally it wouldn't use any water/alcohol at all.
But when going up hills or any time power is required that would drop the maifold vacuum to between 5'' and 10'' of Hg, right about the time preignition or detonation starts to happen water methanol injection would kick on.
Control systems for the water alcohol would be adjustable aftermarket knock sensor, vacuum switch and RPM activated switch.
__________________
1984 chevy suburban, custom made 6.5L diesel turbocharged with a Garrett T76 and Holset HE351VE, 22:1 compression 13psi of intercooled boost.
1989 firebird mostly stock. Aside from the 6-speed manual trans, corvette gen 5 front brakes, 1LE drive shaft, 4th Gen disc brake fbody rear end.
2011 leaf SL, white, portable 240v CHAdeMO, trailer hitch, new batt as of 2014.
|
|
|

|
 04-25-2012, 07:16 PM
04-25-2012, 07:16 PM
|
#138 (permalink)
|
|
Master EcoModder
Join Date: Dec 2010
Location: RI
Posts: 692
Thanks: 371
Thanked 227 Times in 140 Posts
|
Quote:
Originally Posted by t vago

Quote:
|
Originally Posted by IamIan
Are you claiming that temperature alone is the only information needed to determine what the expansion ratio will be for saturated steam? ... that no other factors of the context will have any influence at all???
|
That's right. |
Ok ... maybe it's just me being a bit thick or slow on the uptake ... but that seems like a VERY bold claim to me ... so here is the information ... show me your stuff ... you have a container of water and air at 80C what if any is the expansion ratio for the steam if any? ... Because you claim that this temperature information is the only information you need ... I can do anything else I can think of and not have to tell you any bit of the rest of the context details ... and no matter what I do it should have no influence at all on the expansion ratio ... as if there can only ever be one specific expansion ratio for one temperature.
So go ahead ... give me your one specific expansion ratio for this temperature ... the one specific expansion ratio that will hold the same for any and all other context details.
My prediction ... you'll back away from your very bold claim above in some way ... but maybe I'm wrong ... you tell me.
Quote:
Originally Posted by t vago

1. You're quibbling about the ideal gas law. While it is true that no gas perfectly behaves as an ideal gas, the ideal gas law is sufficient for most gases. Not saturated steam, and not for steam in various regions of temperature and pressure.
|
I don't think I'm quibbling about the ideal gas law ... it shows the relationship clearly ... but the point I was making you've already agreed to ... different volume containers will have different pressures for the same 0.96g of liquid water +0.04g of steam ... if you prefer to come to agreement of that same point from some other direction ... I don't see much significance in what path you took to get there.
potato 'pohtahto' kind of thing to me.
Quote:
Originally Posted by t vago

2. You're flat-out wrong about using "since no gas exactly follows the ideal gas law, we can still treat steam as an ideal gas."
|
While your wording would seem to infer that you are quoting me here ... I don't recall ever writing this ... and it doesn't read like something I would write... I don't ever recall writing this which you seem to here be claiming is a quote from me.
So do me a favor ... remind me of which post of mine you got this quote from... maybe that will jog my memory or something.
Quote:
Originally Posted by t vago

The red area shows where you can treat steam as an ideal gas. Note that for most of the saturation line between steam and liquid, there is no red.
|
I think I have a different point of view about the ideal gas law , than it seems to me that you do ...the ideal gas law is not the sum total of all influences ... it describes how ideal gasses would react ... the ideal gas law does not include all other factors of real world substances ... it does not include for example that at some temperature and pressure gasoline will auto-ignite , and that reaction will have significant effects on temperatures and pressures ... it also won't tell you what the flame speed of the reaction will be , or what the specific heats of the materials will be, or if they are exothermic or endothermic , etc... it isn't that the ideal gas law doesn't work for systems that have gasoline in the them ... it is not a universal theory of everything ... if the specific substances you have behave in certain ways some of that is not part of what the ideal gas law describes.
I'm sure if someone made a plot of gasoline and air at different temperatures and pressures ... like that graph of yours for water and steam at different temperatures and pressures ... that there would also be significant parts of the graph that also deviate greatly from what just the ideal gas law would predict without any other considerations of the substances.
Which is what I see that graph of yours showing ... to me , it is showing that if you only looked at the ideal gas law and ignored the other behaviors of the substances involved , you would at some temperatures and pressures get very different results than what just the ideal gas law alone would predict... and to me ... that is completely expected to me ... and in no way invalidates the principles of the ideal gas law itself ... it is not a theory of everything ... and I don't expect it to be. |
|
|

|
 04-25-2012, 07:29 PM
04-25-2012, 07:29 PM
|
#139 (permalink)
|
|
MPGuino Supporter
Join Date: Oct 2010
Location: Hungary
Posts: 1,808
iNXS - '10 Opel Zafira 111 Anniversary Suzi - '02 Suzuki Swift GL
Thanks: 831
Thanked 710 Times in 458 Posts
|
Quote:
Originally Posted by IamIan

Ok ... maybe it's just me being a bit thick or slow on the uptake
|
Yep.
Quote:
Originally Posted by IamIan

... but that seems like a VERY bold claim to me ... so here is the information ... show me your stuff ... you have a container of water and air at 80C what if any is the expansion ratio for the steam if any?
|
Specific volume of liquid water at 80 C: 0.00103 m^3 / kg
Specific volume of steam at 80 C: 3.40527 m^3 / kg
Expansion ratio at 80 C: 3306.1:1
Vapor pressure of steam at 80 C: 47.4 kPa
Quote:
Originally Posted by IamIan

... Because you claim that this temperature information is the only information you need ... I can do anything else I can think of and not have to tell you any bit of the rest of the context details ... and no matter what I do it should have no influence at all on the expansion ratio ... as if there can only ever be one specific expansion ratio for one temperature.
|
So you don't understand the properties of saturated steam. That's okay, it's hard to wrap your head around it at first, especially if you did not take college-level thermodynamics.
Quote:
Originally Posted by IamIan

So go ahead ... give me your one specific expansion ratio for this temperature ... the one specific expansion ratio that will hold the same for any and all other context details.
My prediction ... you'll back away from your very bold claim above in some way ... but maybe I'm wrong ... you tell me.
|
I wouldn't say that maybe you're wrong. I would say that you simply don't understand how water behaves. |
|
|

|
 04-25-2012, 07:37 PM
04-25-2012, 07:37 PM
|
#140 (permalink)
|
|
MPGuino Supporter
Join Date: Oct 2010
Location: Hungary
Posts: 1,808
iNXS - '10 Opel Zafira 111 Anniversary Suzi - '02 Suzuki Swift GL
Thanks: 831
Thanked 710 Times in 458 Posts
|
Quote:
Originally Posted by IamIan

While your wording would seem to infer that you are quoting me here ... I don't recall ever writing this ... and it doesn't read like something I would write... I don't ever recall writing this which you seem to here be claiming is a quote from me.
So do me a favor ... remind me of which post of mine you got this quote from... maybe that will jog my memory or something.
|
Grow up.
Quote:
Originally Posted by IamIan

Technically no gas 100% follows the ideal gas law ... because no gas is actually 100% ideal... but the basic principle of the relationships shown in the ideal gas law do still apply ... even to saturated steam.
|
You spent an entire post that all but stated that explicit statement, and now you appear to be taking offense when I summarize your faulty position within quote marks. I never stated that you made that particular statement, but again, you devoted a novelette to expressing that idea, that steam could somehow be treated as an ideal gas because the ideal gas doesn't perfectly model the behavior of real gases.
|
|
|

|
|