 04-12-2025, 11:15 AM
04-12-2025, 11:15 AM
|
#391 (permalink)
|
|
Master EcoModder
Join Date: Jan 2008
Location: Sanger,Texas,U.S.A.
Posts: 16,514
Thanks: 24,517
Thanked 7,436 Times in 4,817 Posts
|
' IF '
Quote:
Originally Posted by Logic

IF
the temperatures in top the combustion chamber equaled the temperatures of the cylinder walls
THEN
What would happen?
[/B] Are you now saying that you are unaware of quench distances from the cylinder and
[/B]despite the links, believe engine piston sleeves and lube run at 1600F?
OR
Do you just want any readers of this post to think so.
Sleeves usually run at around 140C
and DO NOT go above 170C
Do you need a hand opening the linked research???
Say if it's beyond you and I'll paste in the pictures for you.
I know how much you hate the pictures. (for all to see)
 And yes; there is enough water/steam/vapour moisture both above and below the piston to turn any Boric Oxide back into Boric Acid.
And yes; there is enough water/steam/vapour moisture both above and below the piston to turn any Boric Oxide back into Boric Acid.
But nice work at discombobulating any other readers.
I'm sure it works/worked on many of them.
Only 1 in 20 people has an IQ over 120 after all...
So you've more than earned your pay and no chance of being kicked out of the 'Keep X,Y and Z quiet and make people add weight to their cars around town in an effort to save fuel' club.
Congrats!!! 
So again:
Have YOU tested it?
Because until then, you don't REALLY know...
and
Given an engine to test it on; would you test it OBJECTIVELY??
As you refuse even to answer this questions;
it's pretty obvious what you are actually doing here..! |
--------------------------------------------------------------------------------------
1) The combustion products can reach temperatures ,twice the melting point of a cast iron cylinder ( 4500-F / 2200-F ), so, in this situation, the engine block would melt.\
Gases across the exhaust valve, 3000-F
Valve stem, 1250-F to 1175-F
2) The engine is liquid-cooled by the water/glycol coolant. General Motors allows up to 284-F oil, 239-F coolant., so, no, I do not believe that this area is at 1,600-F, it's just 'exposed' to up to 4,500-F.
3) The top-ring is at 500-F to 300-F / cylinder, 700-F to 200-F
4) It's important for the reader to understand temperatures experienced inside the combustion chamber, within the context of this area being a source of 'moisture' for Boric-Oxide plating maintenance to be maintained, something which doesn't exist since around 2011, according to Dr. Erdemir.
5) Piston skirt operates at 400-F to 200-F
6) Piston pin is at 450-F to 240-F
7) Connecting rod bearing, 400-F to 200-F
8) Bottom cylinder wall, up to 300-F
9) There is no Boric-Oxide present, Erdemir 'screened it' from the program prior to 2011. It never existed as of the 1995 Argonne patent. There's no 'moisture' in the oil 'supplied' to the piston/cylinder. If the walls are at 170-C, that's 70-C above the 'boiling point' of water. 'Emulsifiers in 'Motor oil' are designed to deal with 'condensate' in the oil, which cannot exist above 100-C.
'Condensate' cannot 'form' at 170-C.
Your going to have to 'mathematically' demonstrate the physics of ' enough water/steam/vapor '.
10) I 'know' there's no Boric Oxide in the engine because Dr. Ali Erdemir told us there wouldn't be any, so, the whole issue surrounding available moisture loses all significance.
__________________
Photobucket album: http://s1271.photobucket.com/albums/jj622/aerohead2/
Last edited by aerohead; 04-12-2025 at 11:20 AM..
Reason: add data
|
|
|

|
 Today Today
|
|
|
|
 Other popular topics in this forum...
Other popular topics in this forum...
|
|
|
|
 04-12-2025, 12:00 PM
04-12-2025, 12:00 PM
|
#392 (permalink)
|
|
Master EcoModder
Join Date: Jan 2008
Location: Sanger,Texas,U.S.A.
Posts: 16,514
Thanks: 24,517
Thanked 7,436 Times in 4,817 Posts
|
' Pure PAO '
Quote:
Originally Posted by Logic

Which part am I supposed to weep about??
The pictures?


All the graphs showing decreased friction and wear?
eg:

This last bit??
Benefits Assessment
The boron-based nanolubrication products developed and optimized in this project have
demonstrated high potential to substantially improve the friction, wear, and scuffing performance of base and formulated oils over a range of test conditions.
Optimization of particle size and concentration, as well as the selection of surfactants, was all important for the overall performance of the lubricants and/or additives tested.
Boron-based particulate additives considered in this project included boric acid (H 3BO 3), hexagonal boron nitride (h-BN), boron oxide, and borax.
As part of this project, we also explored a hybrid MoS 2+boric acid formulation for more effective lubrication and reported the results.
These products have the potential to reduce the amount of anti-friction and -wear additives as well as synergistically work with oil additives to significantly improve the durability and long-term performance of many types of machine components that are subjected to the sliding, rolling, reciprocating, and rotating motions that occur in the transportation, manufacturing, oil and gas industry; in chemical and
petroleum plants; and in construction, agriculture, and earth-moving operations.
Overall, these lubrication additives have the potential to provide significant energy, environmental, and economic benefits to many industrial sectors by increasing energy efficiency, productivity, and environmental compatibility and by lowering operational and maintenance costs.
Reduction in friction and wear in mechanical components is expected to translate into higher efficiency, longer durability, and lower emissions in transportation and other cross-cutting industrial applications.
Overall, this project has clearly demonstrated the significant beneficial effect of particulate additives on limiting friction- and wear-related losses.
Improved resistance to scuffing, abrasion, and micropitting is expected to increase resistance to degradation in severe industrial operations involving forming, machining, rolling, rotating, and sliding.
By increasing component life, these lubricants will reduce the need for frequent part replacement and, hence, curtail the consumption of energy that is needed for the manufacturing of new parts.
Realizing such a beneficial impact, industrial partners are seriously considering licensing this technology pending cost-benefit analysis and environmental impact studies.
So again:
Have YOU tested it?
Because until then, you don't REALLY know...
and
Given an engine to test it on; would you test it OBJECTIVELY??
Because I have.
(and just so you know; that will remain the last question asked, no matter how much you try to get it off the last page with multiple long posts.
It simply wont ever 'recede in the rear-view mirror') |
1) Pure PAO isn't a factory-fill lubricant for transaxles, transmissions, transfer-cases, or differentials.
2) 'Boron' research encompassed :
- engine oils
-process oils
-hydraulic fluids
-metal-forming fluids
-cutting fluids
-various greases
3) We're 'limiting' the BORPower discussion to 'engines', nothing else.
A) ' Boron oxide particles were found to be rather hard and somewhat abrasive and, hence, were not considered beyond the initial screening studies.' Dr. Ali Erdemir, page-11, 2013 Report, finished 19-months after the research was concluded.
B) 'particles tend to accumulate in and around the contact spots and thus even block the easy flow of liquid lubricants to these critical spots...' Dr. Ali Erdemir, page- 14, 2013 Report.
C) ' but nanoscale counterparts ( 10-100nm ) 'may' overcome some of these problems ( he said 'may', not 'will' or 'do', as of July 2013, boric acid nanoparticle technology remained an 'unknown quantity' **************
D) 'without surfactants orthoboric acid particles separate out or agglomerate at 100-C ' Dr. Ali Erdemir, page- 23
So, at normal operating automotive engine temperature the particles could block the inlet screen or plug the oil filter, or starve any particular engine component for oil!
E) ' interest in automotive application ', Dr. Ali Erdemir, page- 27
So, it's July, 2013, and Dr. Ali Erdemir is just beginning to think of boric acid nanoparticles in the context of using them in car engines? And within one month, MotorSilk will come out with an additive that was never considered by Dr. Erdemir for automobiles!
F) ' some blends (of PAO-4 with 100-200nm BA ) agglomerated into larger chunks.' Dr. Ali Erdemir, page-38, 2013 Report.
G) So, 'NO', Dr. Erdemir sufficiently scared me off from 'testing' it.
H) The next step in the 'debate' ought to revolve around what actually happened to those reporting on 'BORPower.'
-------------------------------------------------------------------------------------
I'm presently counting seven (7) factual inconsistencies associated with the 'test' experience you reported.
__________________
Photobucket album: http://s1271.photobucket.com/albums/jj622/aerohead2/
Last edited by aerohead; 04-12-2025 at 12:24 PM..
Reason: add data
|
|
|

|
 Yesterday, 06:47 AM
Yesterday, 06:47 AM
|
#393 (permalink)
|
|
Master EcoModder
Join Date: Aug 2022
Location: South Africa
Posts: 836
Thanks: 332
Thanked 339 Times in 295 Posts
|
Quote:
Originally Posted by aerohead

1) Pure PAO isn't a factory-fill lubricant for transaxles, transmissions, transfer-cases, or differentials.
2) 'Boron' research encompassed :
- engine oils
-process oils
-hydraulic fluids
-metal-forming fluids
-cutting fluids
-various greases
3) We're 'limiting' the BORPower discussion to 'engines', nothing else.
A) ' Boron oxide particles were found to be rather hard and somewhat abrasive and, hence, were not considered beyond the initial screening studies.' Dr. Ali Erdemir, page-11, 2013 Report, finished 19-months after the research was concluded.
B) 'particles tend to accumulate in and around the contact spots and thus even block the easy flow of liquid lubricants to these critical spots...' Dr. Ali Erdemir, page- 14, 2013 Report.
C) ' but nanoscale counterparts ( 10-100nm ) 'may' overcome some of these problems ( he said 'may', not 'will' or 'do', as of July 2013, boric acid nanoparticle technology remained an 'unknown quantity' **************
D) 'without surfactants orthoboric acid particles separate out or agglomerate at 100-C ' Dr. Ali Erdemir, page- 23
So, at normal operating automotive engine temperature the particles could block the inlet screen or plug the oil filter, or starve any particular engine component for oil!
E) ' interest in automotive application ', Dr. Ali Erdemir, page- 27
So, it's July, 2013, and Dr. Ali Erdemir is just beginning to think of boric acid nanoparticles in the context of using them in car engines? And within one month, MotorSilk will come out with an additive that was never considered by Dr. Erdemir for automobiles!
F) ' some blends (of PAO-4 with 100-200nm BA ) agglomerated into larger chunks.' Dr. Ali Erdemir, page-38, 2013 Report.
G) So, 'NO', Dr. Erdemir sufficiently scared me off from 'testing' it.
H) The next step in the 'debate' ought to revolve around what actually happened to those reporting on 'BORPower.'
-------------------------------------------------------------------------------------
I'm presently counting seven (7) factual inconsistencies associated with the 'test' experience you reported.
|
LOOOL!  YOU quoted this
YOU quoted this, with a: "Read it and weep!"
 Everyone else:
Everyone else: (as logical deduction seems to be above aerhead)
Boric oxide (B₂O₃) reacts with water (H₂O) to form boric acid (H₃BO₃) a lamellar structure that forms a tribofilm most suitable as a solid lubricant.
Boric Oxide (B₂O₃) i s highly hygroscopic, meaning it readily absorbs moisture from the air to form Boric Acid
So:
If BO all turns into BA: What abrasive BO..???
Here it is! Here it is!! Well... Kind-of...not!: 

That's a chemically inert barrier, making all the oil anti oxidants obsolete.
It's also hard: 85% the hardness of diamond.
IF it is scratched off; the BA reacts with the bare metal in the scratch, re forming the hard, 'rust proof' surface.
Above that is the lamellar (flat) BA platelets forming the smooth surfaces seen here:
 https://ecomodder.com/forum/attachme...1&d=1737635093
Roads are rough and tires treaded because we DON'T want hydrodynamic lubrication (slippery) between our tires and the road when it rains.
ie:
https://ecomodder.com/forum/attachme...1&d=1737635093
Roads are rough and tires treaded because we DON'T want hydrodynamic lubrication (slippery) between our tires and the road when it rains.
ie:
The smoother the surfaces; the better hydrodynamic lubrication works.
(a) is without BA; (b) is with BA:
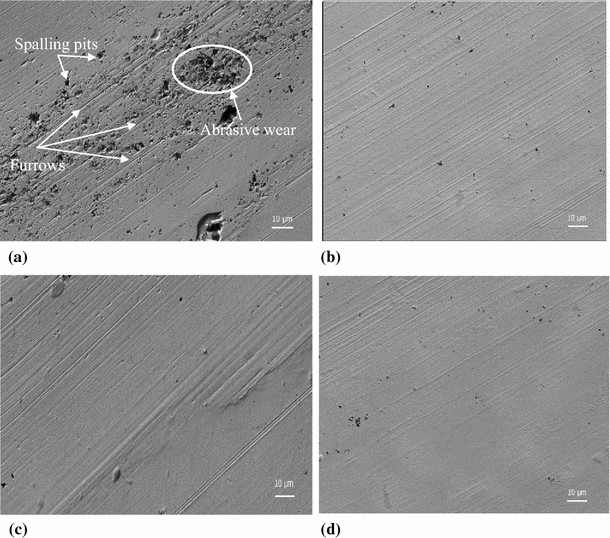
 https://www.sciencedirect.com/science/article/pii/S0301679X22001141
https://www.sciencedirect.com/science/article/pii/S0301679X22001141
But MOST friction (and wear) in an engine is when the piston slows (mixed lubrication) and stops at the end of the strokes. (boundary lubrication)
https://www.sciencedirect.com/topics...ed-lubrication
Here the BA platelets slide over each other as they do for Molybdenum Disulfide and most all other solid lubricants.
The difference With BA is:- The very hard, very chemically inert, self replenishing, 'wear/gap' filling initial surface.
- The resistance to oxidation of the BA lamellar 'platelets' thx to their dependence on moisture, vs Molybdenum Disulfide etc that 'rust'.
- The better coefficient of friction of BA vs Molybdenum Disulfide etc.
Yes there IS WATER in your oil, because Hydrocarbons + Oxygen = Water, at around 1 liter of water formed per liter of fuel burned and blowby.
Around 4ml per minute of water (condensed) ends up in the crankcase and is the reason for the surfactants and antioxidants in engine oils in the 1st place.
Where does it go?
Well it mostly boils and evaporates away, but there is always some in the oil.
https://www.researchgate.net/publica...ection_Systems
'powder will collect and block the oil filter':
BA is soluble in water. (especially hot water)
So at 240ml per hour of water going through the filter what's going to happen?  'powder will collect and block the oil galleries in the crankshaft etc':
'powder will collect and block the oil galleries in the crankshaft etc':
So particles that get through the oil filter will do the same thing..? Why don't they??  Then there's this aerhead ...er... logic:
Then there's this aerhead ...er... logic:  Have you tested it?
No
Have you tested it?
No
Well until you do; you cant ever really know for sure if it works or not can you..?
But I don't have to because it wont work because bla-bla-bla
No; you don't know for sure because you haven't (easily) tested it...
But I do because [tons of huge text blocks to make this logical question recede into the rearview mirror]
 LOL! 
OK:
If you were given an engine to test it on, where you have nothing to lose by doing so; would you test it objectively?
Here we get the flat ignore, with yet more multiple text blocks to make the question disappear into the distance.
Now what does that tell you if not: "Here's a troll trying to very-very hard dissuade anyone from trying it. So hard in fact that I would have to be paid to go to that much effort..!"..???  Now I'll make another logical deduction:
The questions
Now I'll make another logical deduction:
The questions (that would settle the argument, ASSUMING aerhead can be trusted..???) will be ignored, yet again.
Instead we will get many more text blocks full of questions designed to try get me bogged down in inconsequential details and BS yet again.
WHAT WE WILL NOT GET:
Is the logical, conclusion, which is an OBJECTIVE test of BA (dissolved in water) added to an engine's oil. (even a free engine given to him)
Because apparently he (all of a sudden) cant:
measure fuel consumption
and/or
look at smoke, or gas analyze etc.
and/or
test compression,
and/or
note any smoothness (decrease in noise and vibration) and ease of starting,
and/or
note any improvement in the look of the oil with age, as sending samples to a lab is likely an 'impossibility!"
This ( and adding weight to cars) i s the nature of 'our' self appointed 'Economy Savior'

|
|
|

|
 Today, 10:09 AM
Today, 10:09 AM
|
#394 (permalink)
|
|
Master EcoModder
Join Date: Jan 2008
Location: Sanger,Texas,U.S.A.
Posts: 16,514
Thanks: 24,517
Thanked 7,436 Times in 4,817 Posts
|
Argonne's Boric Oxide surface
I believe that it was the folks at The University of Arkansas that mentioned how they actually created the boric oxide coating on their COF test rig. https://www.google.com/search?q=Magn...hrome&ie=UTF-8
--------------------------------------------------------------------------------------
The best I can tell from the existing literature, 'boric-oxide' plating was 'never' created by ANL, inside an internal combustion engine by the introduction of boric acid to the motor oil.
It was never a feature of the US PATENT of 1995, which was the basis for MotorSilk's use of the patent after it expired in 2012.
Some of LOGIC's comments appear to be cribbed directly from MotorSilk's advertising propaganda dating to 2012, one month after Dr. Erdemir stated that Argonne was just then considering boric acid nanotechnology for automotive applications.
MotorSilk and LOGIC are both mistaken in attributing '85% the hardness of diamond' to boric-oxide.
That hardness belongs to cubic lattice-Boron-Nitride, which cannot be created anywhere outside of the likes of General Electric Abrasives, who manufacture it for industrial / commercial abrasive processes.
__________________
Photobucket album: http://s1271.photobucket.com/albums/jj622/aerohead2/
Last edited by aerohead; Today at 10:29 AM..
|
|
|

|
 Today, 10:46 AM
Today, 10:46 AM
|
#395 (permalink)
|
|
Master EcoModder
Join Date: Jan 2008
Location: Sanger,Texas,U.S.A.
Posts: 16,514
Thanks: 24,517
Thanked 7,436 Times in 4,817 Posts
|
' # 392 ( permalink ) '
Congratulations!
You've been nominated for an academy award for your masterful portrayals of both one-trick pony, and broken-record on a phonograph.
----------------------------------------------------------------------------------------
Another congratulation is also in order.
The Select Committee for Solar System Exploration, Space Science Institute, Texas A & M University, has been closely following your brilliant arguments in support of your BORPower testing, and they're so impressed that they've named you top candidate for Astronaut Training at NASA, Houston, for Chief Science Officer on America's first manned space probe mission to the Sun.
Safety studies have passed peer-review and the project has been green-lighted, as the flight will be conducted at night.
__________________
Photobucket album: http://s1271.photobucket.com/albums/jj622/aerohead2/
|
|
|

|
 Today, 11:03 AM
Today, 11:03 AM
|
#396 (permalink)
|
|
Master EcoModder
Join Date: Jan 2008
Location: Sanger,Texas,U.S.A.
Posts: 16,514
Thanks: 24,517
Thanked 7,436 Times in 4,817 Posts
|
' most friction in an engine '
' Two-thirds of friction losses in an engine are estimated to occur during hydrodynamic lubrication'.
Prepared by Energy and Environmental Analysis, Inc., for Oak Ridge National Laboratory, December, 2001.
----------------------------------------------------------------------------------------
' One-third of friction losses in an engine are estimated to occur during boundary region, mixed-film region, and elastohydrodynamic region lubrication'. aerohead, April 14, 2025.
__________________
Photobucket album: http://s1271.photobucket.com/albums/jj622/aerohead2/
|
|
|

|
 Today, 11:58 AM
Today, 11:58 AM
|
#397 (permalink)
|
|
Master EcoModder
Join Date: Jan 2008
Location: Sanger,Texas,U.S.A.
Posts: 16,514
Thanks: 24,517
Thanked 7,436 Times in 4,817 Posts
|
' better COF of BA compared to Molybdenum Disulphide '
That would depend on :
- the 'size' of the BA particles
- the 'concentration' ( by weight ) of the particles
- the test 'load' ( contact pressure ) on the test machine.
- The temperature at which the test was conducted
- Substrate of pin and disk, 440 C steel, 52100 steel, alumina, sapphire ( steel on steel, steel on alumina, alumina-on-alumina, sapphire on steel, sapphire on alumina, sapphire on sapphire etc. )
--------------------------------------------------------------------------------------
In the 1995 Argonne patent, the only 'Motor Oil' tested was SAE 15W-40.
- This baseline oil was...................................... COF= 0.110
- The 1% (wt) nBA-friction- modified 15W-40 was COF= 0.090 ( 18.18% lower ) not 1000%
--------------------------------------------------------------------------------------
- GROUP-III 5W-30 SI with 1.5%(wt) Oleylamide...COF = 0.080 @ 60-C
- RedLine MTL 75W-80...................................... COF= 0.076
- PAO-4 with surfactant & 5% nBA...................... COF- 0.070
- Ford Motor Company SAE 5W-30 with MoDTC......COF = 0.067
- RedLine 10W-30............................................. COF= 0.05517
- RedLine SAE 30W SF........................................COF= 0.054
- RedLine SAE 20W-50....................................... COF= 0.05226
- PAO-4 with 1% nBA......................................... COF= 0.050
- RedLine SAE 40W............................................. COF= 0.043
- PTFE on PTFE.............................................. ..... COF= 0.0200
- RedLine MTL 75W-80......................................... COF= 0.015 @ 800-RPM
- RedLine MTL 75W-80......................................... COF= 0.100 @ 1437-RPM
__________________
Photobucket album: http://s1271.photobucket.com/albums/jj622/aerohead2/
Last edited by aerohead; Today at 12:03 PM..
Reason: add data
|
|
|

|
 Today, 12:11 PM
Today, 12:11 PM
|
#398 (permalink)
|
|
Master EcoModder
Join Date: Jan 2008
Location: Sanger,Texas,U.S.A.
Posts: 16,514
Thanks: 24,517
Thanked 7,436 Times in 4,817 Posts
|
' flat ignore '
Typically, one doesn't put the roof on a house until everything 'underneath' is already constructed.
I've answered your question already, but since ignoring my posts appears to be your modus operandi, you're never aware.
We'll get to the nuts and bolts of 'test' after the preparatory work is concluded.
__________________
Photobucket album: http://s1271.photobucket.com/albums/jj622/aerohead2/
|
|
|

|
 Today, 12:44 PM
Today, 12:44 PM
|
#399 (permalink)
|
|
Master EcoModder
Join Date: Aug 2012
Location: northwest of normal
Posts: 29,353
Thanks: 8,351
Thanked 9,116 Times in 7,526 Posts
|
Quote:
|
Typically, one doesn't put the roof on a house until everything 'underneath' is already constructed.
|
Do that in the PNW in the rainy season and what you get is black mold.
__________________
.
.Without freedom of speech we wouldn't know who all the idiots are. -- anonymous poster
___________________
.
.Impossible is just something we haven't done yet. -- Langley Outdoors Academy
|
|
|

|
|