 04-10-2021, 07:25 PM
04-10-2021, 07:25 PM
|
#1 (permalink)
|
|
Banned
Join Date: Nov 2017
Location: Australia
Posts: 2,060
Thanks: 107
Thanked 1,608 Times in 1,137 Posts
|
Decimal places
I see people here often quoting data to many decimal places. Sometimes, even (as Aerohead did recently), increasing the number of decimal places after doing a calculation.
So what's the issue?
The number of decimal places is indicative of the accuracy of the measurement, with the more decimal places, the higher the degree of assumed accuracy.
Two points.
1. You cannot increase the number of decimal places that was present in the original measurement. So for example, a 9 per cent reduction in a drag coefficient of 0.32 cannot become 0.2912 - there's no basic of validity for the last two decimal places (ie there was no such resolution in the original measurement) and so it becomes 0.29.
2. As textbook I have says of the use of too many decimal places: "They imply a very precise result from imprecise data." Therefore, the number of decimal places should reflect the uncertainty in the original measurements. If I do fuel economy measurements over a relatively short distance (i.e. not thousands of km) and get 3.2 litres/100 km, and then make a change and get 2.9 litres/100km, the improvement is 9.375 percent. But realistically, taking into account the uncertainties involved, it's better to say "about 10 per cent".
As soon as someone starts using lots of decimal places, you know they either have incredibly precise measurements - or they don't have a good feel for the data.
|
|
|

|
 Today Today
|
|
|
|
 Other popular topics in this forum...
Other popular topics in this forum...
|
|
|
|
 04-10-2021, 09:40 PM
04-10-2021, 09:40 PM
|
#2 (permalink)
|
|
Master EcoModder
Join Date: Aug 2012
Location: northwest of normal
Posts: 29,344
Thanks: 8,347
Thanked 9,111 Times in 7,524 Posts
|
You're not wrong.
en.wikipedia.org/wiki/Accuracy_and_precision
Quote:
In a set of measurements, accuracy is closeness of the measurements to a specific value, while precision is the closeness of the measurements to each other.
[snip]
In simpler terms, given a set of data points from repeated measurements of the same quantity, the set can be said to be accurate if their average is close to the true value of the quantity being measured, while the set can be said to be precise if the values are close to each other. In the first, more common definition of "accuracy" above, the two concepts are independent of each other, so a particular set of data can be said to be either accurate, or precise, or both, or neither.
|
__________________
.
.Without freedom of speech we wouldn't know who all the idiots are. -- anonymous poster
___________________
.
.Impossible is just something we haven't done yet. -- Langley Outdoors Academy
|
|
|

|
|
The Following User Says Thank You to freebeard For This Useful Post:
|
|
 04-10-2021, 11:34 PM
04-10-2021, 11:34 PM
|
#3 (permalink)
|
|
Moderator
Join Date: Feb 2012
Location: Urbana, IL
Posts: 1,939
Thanks: 199
Thanked 1,807 Times in 943 Posts
|
This is an important point. First semester of an engineering or sciences degree, multiple professors will spend class time on this (in the degree I'm finishing now, we covered this in Chemistry 101, Physics 141, and Engineering Science 201. Yes, it was duplicative. Yes, it's that important).
This is the concept of "significant figures," or, as we referred to it in school, "sig figs." The basic rules are:
1) The last digit is uncertain--it's an estimate. Measure something from a tape measure marked in centimeters, and you estimate the decimal point (between the marks), eg 23.7 cm. Measure it with a tape marked in millimeters, and the decimal point is again estimated, eg 236.7 mm. Accuracy depends on the measuring device, but in all cases the last digit--just beyond the resolution of the device--is estimated. It's uncertain.*
2) Addition and subtraction: The uncertain digit is taken from the smallest significant figure of the two numbers, eg 0.067 + 1.40 = 1.467.
3) Multiplication and division: The answer is rounded to the smallest number of significant figures of the input data, eg 0.067 * 1.40 = 0.094 (not 0.0938; one number has only two significant figures, so the answer is rounded to two significant figures).
*Note that this says nothing about the calibration of the device. If you use a tape marked in millimeters but each mark is actually 1.1 mm, you're going to be way off regardless of correct rounding.
|
|
|

|
|
The Following 2 Users Say Thank You to Vman455 For This Useful Post:
|
|
 04-11-2021, 01:24 AM
04-11-2021, 01:24 AM
|
#4 (permalink)
|
|
Master EcoModder
Join Date: Aug 2012
Location: northwest of normal
Posts: 29,344
Thanks: 8,347
Thanked 9,111 Times in 7,524 Posts
|
Significant figures are significant.
You can add all the decimal places you want so long as they're all zeros.
__________________
.
.Without freedom of speech we wouldn't know who all the idiots are. -- anonymous poster
___________________
.
.Impossible is just something we haven't done yet. -- Langley Outdoors Academy
|
|
|

|
|
The Following User Says Thank You to freebeard For This Useful Post:
|
|
 04-11-2021, 08:11 AM
04-11-2021, 08:11 AM
|
#5 (permalink)
|
|
Moderator
Join Date: Feb 2012
Location: Urbana, IL
Posts: 1,939
Thanks: 199
Thanked 1,807 Times in 943 Posts
|
Quote:
Originally Posted by freebeard

Significant figures are significant.
You can add all the decimal places you want so long as they're all zeros.
|
Only if you mean zeros before other digits, eg 0.000937 has three significant figures but 0.937000 has six. |
|
|

|
|
The Following User Says Thank You to Vman455 For This Useful Post:
|
|
 04-11-2021, 10:19 AM
04-11-2021, 10:19 AM
|
#6 (permalink)
|
|
Master EcoModder
Join Date: Dec 2011
Location: New Zealand
Posts: 5,141
Thanks: 2,928
Thanked 2,603 Times in 1,619 Posts
|
Others have covered it pretty thoroughly already, but I'd like to put out there that, after a calculation, you can have more decimal places, even while following the rules for significant figures. It isn't the number of decimal places that matters, but rather, maintaining the precision of measurement through the calculations.
|
|
|

|
|
The Following User Says Thank You to Ecky For This Useful Post:
|
|
 04-11-2021, 02:48 PM
04-11-2021, 02:48 PM
|
#7 (permalink)
|
|
Long time lurker
Join Date: May 2019
Location: Uk
Posts: 218
Thanks: 110
Thanked 153 Times in 119 Posts
|
resolution
Another point is the resolution of the original measurements.
" If I do fuel economy measurements over a relatively short distance (i.e. not thousands of km) and get 3.2 litres/100 km, and then make a change and get 2.9 litres/100km, the improvement is 9.375 percent. But realistically, taking into account the uncertainties involved, it's better to say "about 10 per cent".
"
If you read 3.2, you are really reading 3.2 +/- 0.05, and 2.9 +/- 0.05. While the reading says 3.2, it could actually be 3.24999, or it could be 3.1500. So your change could be anywhere between 6.4% and 12.4%, (if you assume 3.25 and 2.95, and compare to 3.25 and 2.85) so the margin of error on your 9% is plus minus 33%.
|
|
|

|
|
The Following User Says Thank You to AeroMcAeroFace For This Useful Post:
|
|
 04-11-2021, 05:14 PM
04-11-2021, 05:14 PM
|
#8 (permalink)
|
|
Banned
Join Date: Nov 2017
Location: Australia
Posts: 2,060
Thanks: 107
Thanked 1,608 Times in 1,137 Posts
|
Yep, I was trying to make it all easy!
For example, the textbook I am using has three different rules for sig figs and uses no less than 10 examples, with sig figs up to 6.
What I was attempting to do is address the most egregious examples of people quoting data and calculations here as if they were quite precise, when clearly they are not.
|
|
|

|
 04-12-2021, 10:35 AM
04-12-2021, 10:35 AM
|
#9 (permalink)
|
|
Eco-ventor
Join Date: Oct 2010
Location: sweden
Posts: 1,647
Thanks: 77
Thanked 713 Times in 452 Posts
|
Quote:
|
Only if you mean zeros before other digits, eg 0.000937 has three significant figures but 0.937000 has six.
|
But what about when the number is 937000?
__________________

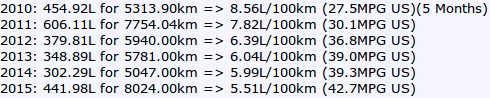
2016: 128.75L for 1875.00km => 6.87L/100km (34.3MPG US)
2017: 209.14L for 4244.00km => 4.93L/100km (47.7MPG US)
|
|
|

|
|
The Following User Says Thank You to jakobnev For This Useful Post:
|
|
 04-12-2021, 11:23 AM
04-12-2021, 11:23 AM
|
#10 (permalink)
|
|
Somewhat crazed
Join Date: Sep 2013
Location: 1826 miles WSW of Normal
Posts: 4,564
Thanks: 596
Thanked 1,253 Times in 1,105 Posts
|
I'm going sociology here: more than 2 places past the decimal is meaningless (mostly because you cant half a person and expect meaningful results)
I doubt that the homebuilt state of the art can actually measure to that accuracy without compensation or under closed controlled atmospheres. Go ahead and prove me wrong with actual data and not massaged findings.
|
|
|

|
|